Nanotechnology in Dentistry: Dental Implants
In the past few years, nanotechnology research work has focused on the production of dental and biomedical materials that possess antimicrobial activities and tailored functions. Silver nanoparticles have been subject to rigorous research for their potential applications in restorative dentistry, orthodontics and oral implantology. The undergoing research work includes laboratory testing on several bacterial strains.
Titanium dental implants doped with silver nanoparticles are tested in vitro against bacterial species isolated from infected peri-implant sites to assess their antimicrobial power. The production of such implants must consider the design of implants as well as the concentration and synthesis of each substituent. It was found that a concentration of 0.05 ppm silver ions could provide the desired antimicrobial effect; but higher concentration (0.1 ppm of silver) can be highly toxic to the surrounding cells.
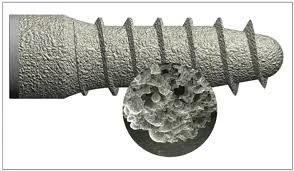
There are several synthesis (silver incorporation) methods reported in literature, such as implantation, electrophoresis, sol-gel, ion exchange and plasma spray. Synthesis methods need to be simple, inexpensive and resulting in uniform appearance of silver ions with specific particle size (25–450 nm). Synthesis time could have significant effect on surface properties of the implants, that is, the longer silver deposition process, the higher surface roughness and less wettability.

Rough and dry surfaces can be explained as a result of longer synthesis time which allows more silver particles to precipitate forming larger clusters. Roughness is an essential issue when dealing with dental implants and osteo-integration. It is well understood that roughness enhances osteo-integration; but, on the other hand, bacterial biofilm formation increases over rough surfaces. Too much surface roughness is undesirable, especially for non-submerged implants which have the coronal parts lying on the gum. Particles size is influenced by several factors such as pH, reaction time, addition of other elements and the buffering conditions.
Wettability is important for cell adhesion to the implant’s surface, and hydrophobic surfaces are unfavorable in implant dentistry as they provide good medium for bacterial attachment. Wet surfaces have additional advantage as they promote better adhesion of osteoblasts and fibroblasts, which, in turn, promotes osteo-integration. They also neutralize surface roughness that might occur during the synthesis process. However, the balance between roughness and wettability is still a subject under rigorous evaluation. It is clearly evident that there are several factors that contribute to implant integration with bone and gum tissues.
Peri-implantitis continues to be the most threat for successful implant treatment due to the dynamic ecosystem of colonizing microorganisms, low blood supply and absence of periodontal space between the implant and the surrounding tissues. Synthesis process and subsequent dissolution of nanoparticles have crucial action on the disruption of the bacterial cells’ membranes and the release of their intracellular biomolecules. The antibacterial action is promoted through several mechanisms such as incorporating metal-oxides that kill bacteria by damaging their intracellular structures, disruption of the cell signals, changing cell motility and DNA replication and subsequent suicidal (apoptotic death). The silver ions can also cause the bacteria to undergo a non-growing/non-culturing state as a result of respiration arrest.
Laboratory studies have shown that P. gingivalis, E. coli and S. aureus were the most sensitive species to silver nanoparticles, while streptococci and S. mutans were the most resistant. Gram-negative bacteria were also resistant to silver nanoparticles as they have slow metabolism and different nature/thicknesses of the cell wall. The antimicrobial effects of silver nanoparticles change over time, they could be stronger in the first hours of incubation in the medium; they are also dependent on the percentage weight of silver ions and the method used for nano-silver incorporation as both contribute to stable ion release. Titanium implants can be manufactured to allow higher release of the antibacterial agent at the space surrounding the implant over short time to induce the required cytotoxicity. However, there is an extensive, heterogeneous medium of microbial species and phylotypes in the oral cavity with switch ability from hydrophobic to hydrophilic when changes in environment occur.

It is worth mentioning that silver nanoparticles, although considered as biocompatible materials, still carry the risk of cytotoxicity, especially for fibroblasts and osteoblasts. This is governed by the concentration of silver ions, but there is no clinical study confirming the absolute biocompatibility or toxicity thresholds. Cytotoxicity, or human cells’ death, occurs in a similar manner to the above-mentioned mechanism of bacteriolysis. It is also dependent on the surface area, size/shape/purity of silver ions, protein adsorption and suspension stability.
The current research work on silver nanoparticles shows that they possess a time-limited antimicrobial release which could be from weeks to months after implantation then the bactericidal concentrations keep at a lowr level. Antimicrobial effects can be the same among similar ranges of silver ions’ concentrations.
Silver nanoparticles have thus gained much popularity in dental industry but more research work is needed to assess the adequate use of these particles as an additive to titanium implants to inhibit bacterial growth and infection.